Neurocardiology emerged as an interdisciplinary research field in the 80 s, well after Nelson Mandela wrote his famous quote on 1 January 1976: 'A good brain and a good heart are always a formidable combination'. Yet, our knowledge of brain–heart interactions, especially those involving regulatory RNAs, remains in a rudimentary stage.[1]
Of all the organs in the human body, the brain and heart are examples of high energy-demanding tissues that require synchronous action potentials for normal functioning. Consequently, both organs are highly susceptible to and share common risk factors for, vascular complications. The relationship between the brain and heart in various neurocardiogenic syndromes, including vascular-, metabolic-, or electrical disorders, is a topic of discussion.[2] In patients with COVID-19, cardiovascular and neurological comorbidities are associated with high mortality and with (epi)transcriptomic changes affecting the brain–heart axis.[3] Regulatory RNAs including, but not limited to non-coding RNAs, are involved in brain and heart diseases and show some potential as biomarkers in both conditions.[4] For instance, microRNAs and circular RNAs are potential biomarkers of neurological outcome after cardiac arrest.[5,6] The therapeutic and biomarker potential of RNAs in the brain–heart axis is a topic of ongoing discussions within the EU-CardioRNA COST Action network (www.cardiorna.eu).[1,7]
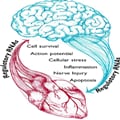
Parkinson's disease (PD), the second most common neurodegenerative disease, begins with the loss of dopaminergic neurons in the mid-brain. Seemingly benign early symptoms quickly extend past the brain and eventually transform into debilitating systemic disorders including tremors, issues with normal bodily movements, a severe reduction in quality of life, and ultimately death. The treatment of PD remains symptomatic. Levodopa, one of the most commonly administered drugs for the symptomatic treatment of PD is also known to cause serious side effects, including cardiovascular defects. Further, it is known that PD sufferers have an elevated risk of developing cardiovascular disease (CVD) due to shared pathogenesis and risk factors. Age is the common risk factor for both CVD and PD development. Factors such as coffee consumption, and physical activity are associated with a lower risk of both PD and CVDs. In addition, males are more susceptible to developing these age-related conditions.[8] Along with the common risk factors, several dysregulated pathways connect the etiologies of CVD and PD. Perturbation in the Ca2+ intake causing disturbances in the action potential, cellular stress causing mitochondrial dysfunction and increased production of reactive oxygen species, cellular inflammation, nerve injury, and apoptosis are some of the mechanisms dysregulated in both these pathological conditions (Figure 1).[8] These evidences strengthen the link between PD and CVD and further highlight the need to study the functional links between neurodegeneration and cardiovascular risk.
Figure 1.
Bidirectional role of regulatory RNAs in common brain–heart mechanisms (illustration ©LIH).
Recent research focusing on specific classes of RNA molecules may be able to help researchers with this endeavor. In the brain, these regulatory RNAs are involved in the regulation of gene expression-controlling neuronal and synapse maintenance, regulation of cellular stress, and apoptosis.[8] In the heart, these act as gene expression and cell cycle regulators facilitating normal cardiac functioning and regeneration.[4] They serve as important regulators of normal physiological function and disease pathogenesis in both the brain and heart further emphasizing the significance of RNAs in maintaining a healthy brain and heart. In this line, regulatory RNAs, particularly microRNAs, are circulating and act as paracrine mediators, hence are potential biomarkers and candidates to study functional interactions between the two organs, specifically during cardio-neural disturbances associated with PD.[9] With this in mind, it would be pertinent to study the role of regulatory RNAs as well as (epi)transcriptomic mechanisms in the development of CVD in PD. For instance, cardiac-specific microRNAs could be studied in PD to see whether their expression profiles change in the PD state and therefore whether they could hold potential to be used as a biomarker or novel treatment in neurocardiogenic conditions. Indeed, microRNAs regulate several pathways common between neurological and cardiac diseases, such as inflammation, oxidative stress, and apoptosis.[9]
Leucine-rich repeat kinase-2 (LRRK2) is a gene associated with high-risk a genetic form of PD. LRRK2 is localized in the mitochondria and is known to regulate mitochondrial function in high-energy demanding organs such as the brain and the heart. Indeed, it has been observed that patients with idiopathic PD exhibit diminished heart rate variability, while those with LRRK2-associated PD exhibit normal heart rate regulation.[10] In addition, the deficiency of LRRK2 in the heart was shown to be protective against in vivo cardiac remodeling.[11] These studies show a contrasting role of LRRK2 in the brain and heart, and support LRRK2 as a potential disease-modifying target for therapy. Overall, such research underlines the need for further investigation to be conducted looking into the risk of CVD in various forms of PD in large-scale, multicenter patient cohorts. Moreover, this is a call to arms for researchers to consider a systemic approach in the combat against PD. To date, there are no disease-modifying therapies available for PD. A comprehensive approach including different modalities will help to uncover the biological mechanisms behind the link between PD and CVD so that more effective, personalized treatment strategies can be offered to PD patients suffering from CVD.
In this regard, regulatory RNAs may prove to bridge CVD and PD and could hold the key to novel therapeutic discovery. By targeting regulatory RNAs, we may get to the heart of PD.
Funding
This work has been funded by grants from the Fonds National de la Recherche (FNR) of Luxembourg to S.A. (grant # AFR 14566210) and to Y.D. (grants # C14/BM/8225223, C17/BM/11613033, COVID-19/2020-1/14719577/miRCOVID). Y.D. is funded by the Ministry of Higher Education and Research of Luxembourg, the Heart Foundation—Daniel Wagner.
Eur Heart J. 2023;44(23):2059-2060. © 2023 Oxford University Press
Copyright 2007 European Society of Cardiology. Published by Oxford University Press. All rights reserved.