Abstract and Introduction
Abstract
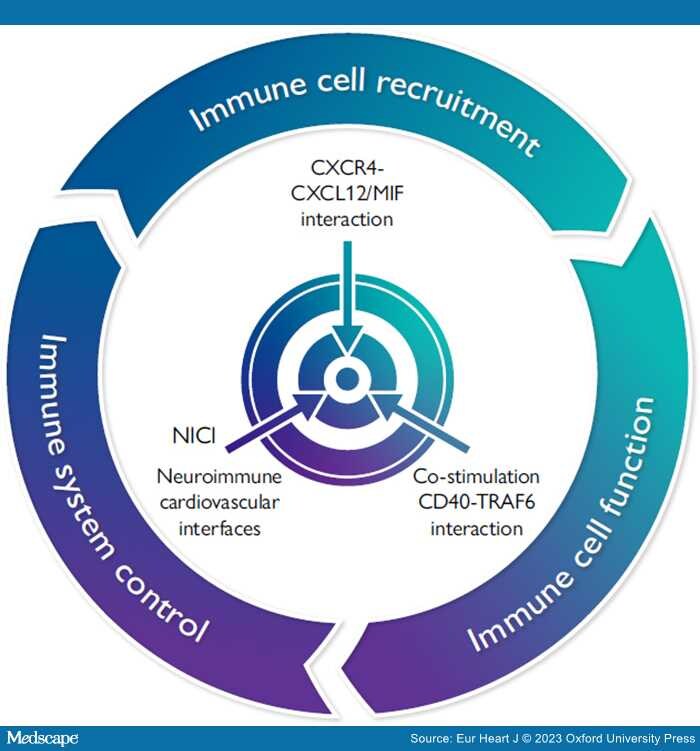
Graphical Abstract
To better treat the residual risk conferred by inflammation in atherosclerosis, a triad of three novel strategies can be envisioned. Immune cell recruitment can be targeted by specific interference with the CXC chemokine receptor (CXCR4) axis and its ligands CXCL12 or MIF. Immune cell function can be modulated by selective targeting the interaction between the co-stimulatory molecule CD40 and the TNF-receptor associated factor TRAF6. Recently identified neuroimmune cardiovascular interfaces regulating atherosclerosis and their connectivity in neuronal circuits could be specifically targeted by surgical, pharmaceutical, or bioelectronics methods.
This review based on the ESC William Harvey Lecture in Basic Science 2022 highlights recent experimental and translational progress on the therapeutic targeting of the inflammatory components in atherosclerosis, introducing novel strategies to limit side effects and to increase efficacy. Since the validation of the inflammatory paradigm in CANTOS and COLCOT, efforts to control the residual risk conferred by inflammation have centred on the NLRP3 inflammasome-driven IL-1β-IL6 axis. Interference with the co-stimulatory dyad CD40L–CD40 and selective targeting of tumour necrosis factor-receptor associated factors (TRAFs), namely the TRAF6–CD40 interaction in macrophages by small molecule inhibitors, harbour intriguing options to reduce established atherosclerosis and plaque instability without immune side effects. The chemokine system crucial for shaping immune cell recruitment and homoeostasis can be fine-tuned and modulated by its heterodimer interactome. Structure–function analysis enabled the design of cyclic, helical, or linked peptides specifically targeting or mimicking these interactions to limit atherosclerosis or thrombosis by blunting myeloid recruitment, boosting regulatory T cells, inhibiting platelet activity, or specifically blocking the atypical chemokine MIF without notable side effects. Finally, adventitial neuroimmune cardiovascular interfaces in advanced atherosclerosis show robust restructuring of innervation from perivascular ganglia and employ sensory neurons of dorsal root ganglia to enter the central nervous system and to establish an atherosclerosis-brain circuit sensor, while sympathetic and vagal efferents project to the celiac ganglion to create an atherosclerosis-brain circuit effector. Disrupting this circuitry by surgical or chemical sympathectomy limited disease progression and enhanced plaque stability, opening exciting perspectives for selective and tailored intervention beyond anti-inflammatory strategies.
Introduction
Atherosclerosis is a chronic inflammatory disease of the arterial wall and the pathophysiological substrate of acute coronary syndromes and ischaemic strokes,[1] which account for the major cause of mortality and disability worldwide.[2,3] The clinical management of atherosclerosis and its complications has considerably improved due to recent developments in revascularization techniques and preventive strategies, mainly featuring effective lipid-lowering therapeutics (i.e. statins and PCSK9 inhibitors).[4] Notwithstanding, the prevalence of cardiovascular disease and the incidence of acute events (e.g. myocardial infarction) has increased over the last 30 years with a high burden of mortality,[2] mandating a quest to identify novel targets to reduce the residual risk of cardiovascular events. This need is illustrated by the global burden of disease study.[2,3] Herein, cardiovascular disease, which is overwhelmingly caused by atherosclerosis as the underlying pathology, is the leading cause of mortality, claiming 15.6 million lives in 2010. Compared with other entities, this prevalence will continue to dominate, owing to an increasing life expectancy in western, but also in emerging societies. In the EU, atherosclerotic cardiovascular disease and associated thrombosis represent the most frequent cause of death, accounting for 40% or 2 million per year. The enormous socioeconomic costs imposed by coronary artery disease (CAD) on European healthcare systems are estimated at 110 billion Euro per year and continue to rise. Not only is atherothrombosis-based cardiovascular disease the underlying cause of heart disease and stroke, it also predisposes to an increased risk for lethal outcomes in patients with COVID-19.[5] Vice versa, COVID-19 increases the risk for disseminated thrombotic disease, due to excessive inflammation, platelet activation, and endothelial dysfunction.[6,7] Hence, anti-inflammatory therapeutic strategies may not only target atherosclerosis, atherothrombosis, and bone marrow homoeostasis as factors in cardiovascular disease, but they may also be of benefit to treat COVID-19-related complications.
Since the PROVE-IT and JUPITER trials, an additive prognostic value of high-sensitivity C-reactive protein (hsCRP) and the putative benefit of anti-inflammatory therapy has been postulated.[8] Given the importance of inflammation in atherosclerosis, anti-inflammatory agents have been tested in large randomized clinical trials. The positive outcome of the CANTOS trial, which randomized patients with previous myocardial infarction and elevated CRP to canakinumab, an antibody blocking the cytokine IL-1β, and resulted in a significant risk reduction, has validated the inflammatory pathogenesis of atherosclerosis. Likewise, the COLCOT and LoDoCo trials, which showed a CRP-independent effect of anti-inflammatory, low-dose colchicine in stable disease, constitute an important breakthrough in attempting to combat residual inflammation.[9–11]
These results were contrasted by the negative yet informative CIRT trial that tested low-dose methotrexate, a broad immunosuppressive agent, illustrating the complexity and specificity of atherosclerotic inflammation.[12] The moderate effect size, high cost, and noticeable side effects, namely in aged and diabetic patients, explained why efforts to pursue canakinumab for entry into clinical practice were discontinued, while related issues prevent colchicine despite being more affordable from becoming a blockbuster. Multiple studies to target the NLRP3 inflammasome, an integral activator of IL-1β production, which is implicated as a metabolic sensor and central relay in atherogenesis triggered by cholesterol crystals, disturbed flow, neutrophils extracellular traps (NETs), age/clonal haematopoiesis, or somatic mutations are underway.[13,14] Yet, blocking NLRP3 is expected to encompass considerable pleiotropy and immunological side effects, so that selective targeting of upstream stimulators may be more feasible and ideal. This will however require deeper exploration of immunomodulatory pathways more specific to atherosclerosis, in a persistent quest for alternative inflammatory targets and more specific therapeutics with limited side effects and improved precision in chronic treatment.[15] Our endeavour to identify some of these targets, to establish approaches for the development of novel modulatory agents, as well as the discovery of alternative pathways involving neuroimmune cardiovascular interfaces and thereby shaping the inflammatory pathogenesis of atherosclerosis—has been presented in the 2022 ESC William Harvey Lecture on Basic Science and is the topic of this article (Graphical Abstract).
Eur Heart J. 2023;44(29):2672-2681. © 2023 Oxford University Press
Copyright 2007 European Society of Cardiology. Published by Oxford University Press. All rights reserved.