Abstract and Introduction
Abstract
Purpose of Review: Gastroesophageal reflux disease (GERD) is the most common chronic condition with increasing prevalence in the Western world. Despite medical therapy, a considerable proportion of patients continue to experience symptoms, thus fueling the demand for minimally invasive GERD treatment options. This review will assess the currently available endoscopic approaches by analyzing their outcomes data, indication for use and limitations.
Recent Findings: With increasing evidence of the safety and efficacy of endoscopic therapies, recent guidelines and consensus society documents have updated their recommendations for the endoscopic treatment of GERD. In this review, we have comprehensively assessed the current landscape of endoscopic approaches for the treatment of GERD and provided insight into future directions.
Summary: Endoscopic therapies for GERD show promise as new treatments emerge and existing therapies evolve into safer and more reproducible options. They are well positioned to cater to a large subset of the population suffering from chronic condition of GERD.
Introduction
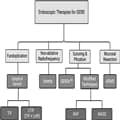
Gastroesophageal reflux disease (GERD) is a chronic condition that can significantly impact patients' quality of life. It can present with typical (e.g., heartburn, regurgitation, bloating) and atypical (e.g., cough, hoarseness, breathing difficulty) GERD symptoms. The prevalence of GERD varies globally but has been increasing over the years. A systematic review of population-based studies showed that the prevalence of GERD is 15.4% in North America, 19.6% in Central America, 17.6% in South America, 21.3% in Southern Europe, 15.5% in Northern Europe, 14.1% in Australia, 15% in the Middle East, 22.1% in South Asia and 7.4% in Southeast Asia.[1] Lifestyle modifications and medical therapy are the first-line treatment for GERD. Proton pump inhibitors (PPIs) are the cornerstone of medical treatment. However, a large portion of patients with chronic GERD remain refractory to medical therapy.[2] Moreover, an additional subset of patients, who, despite being candidates for long-term medical therapy, are not inclined towards pursuing this course of treatment. On the other hand, antireflux surgery (ARS) has shown effectiveness in patients with chronic/refractory GERD but is more invasive and associated with long-term adverse effects like dysphagia, uncontrolled flatulence, and gas bloating.[3,4] Endoscopic minimally invasive GERD therapy has the potential to bridge this gap between medical and more invasive ARS. With the mounting evidence regarding the effectiveness and safety of endoscopic therapy over the years, it has been included in the latest guideline recommendations with ARS, as summarized in Table 1. The aim of this review is to summarize the current landscape of endoscopic therapies and highlight future directions. Figure 1 shows an overview of the endoscopic therapies we will briefly cover in our review.
Figure 1.
Overview of the prominent endoscopic therapies. ARMS, antireflux mucosectomy; cTIF, concomitant TIF; LHR, laparoscopic hernia repair; MASE, mucosal ablation and suturing of esophagogastric junction; RAP, resection and plication; TIF, transoral incisionless fundoplication.
Transoral Incisionless Fundoplication
Transoral incisionless fundoplication (TIF) is a minimally invasive endoscopic fundoplication technique performed using the EsophyX device (EndoGastric Solutions, Redmond, WA). It was initially approved in 2007, and since then, the device and the procedure have evolved over the years. The current procedural and device iterations are TIF 2.0 and EsophyX-Z, respectively. TIF is anatomically and physiologically similar to surgical fundoplication. TIF creates a full-thickness gastro-gastric plication by folding the gastric fundus up and around 2–4 cm length of the distal esophagus, which has been retracted below the diaphragm and anchored with polypropylene fasteners. It creates a 270–300° fundoplication while the device's diameter controls the flap valve luminal diameter, preventing over-tightening. This allows gas to still escape from the esophagus, minimizing gas-bloat postprocedure. Dysphagia, bloating, and flatulence have been reported more frequently with ARS compared to medical therapy in the 5-year randomized, open, parallel-group trial in Europe, the LOTUS trial.[4]
A meta-analysis of three randomized controlled trials showed that TIF improved objective (decreased esophageal pH) and subjective (decreased utilization of PPI, patient satisfaction, and improved quality of life) parameters for GERD compared to a sham procedure or PPI.[10–13] The 5-year data from a randomized controlled trial, TEMPO trial, and long-term data from a meta-analysis by Testoni et al. in 2021 provide evidence for the durability of the procedure.[14,15] In addition, the efficacy of TIF has been assessed in multiple noncomparative studies.[16–23]
The combined serious adverse event rate for all iterations of TIF range from 2% to 2.5%.[24,25] However, to accurately assess the adverse effect profile, it is essential not to group different devices and techniques. Since the introduction of the TIF 2.0 technique, the serious adverse events have lowered to approximately 0.4–1%.[26–28] There is a paucity of high-quality data directly comparing ARS to TIF. Even though concerns have been raised on the methodology of the analysis but a network meta-analysis compared TIF with laparoscopic Nissen fundoplication (LNF) or PPI showed a higher probability of LNF decreasing the acid exposure. In comparison, TIF had a higher probability of improving subjective symptoms.[29] It is safe to conclude from a review of the available evidence that TIF is safe, effective, and durable in selected patients with GERD.
Generally, ideal candidates for TIF include patients with a confirmed diagnosis of GERD with lower esophageal sphincter incompetence (Hill grade II or less) without hiatal hernia defect >2 cm. Given the ideal candidacy exclude a large proportion of patients, concomitant hiatal hernia repair with TIF (cTIF) was introduced, which also addressed the concerns for recurrent symptoms after TIF for patients with Hill grade >2 and hiatal hernia size >2 cm.[21,30,31] In 2017, FDA allowed modification of the TIF device instructions for use, permitting TIF to be performed with hiatal hernia repair, similar to surgical fundoplication. There is growing evidence of the safety and efficacy of cTIF procedures for typical and atypical GERD symptoms.[26,32–34] A recent study by Jaruvongvanich et al. compared hiatal hernia repair followed by LNF to cTIF and showed comparable results between the two groups for postprocedural PPI use, dysphagia, esophagitis, disrupted wrap, and hiatal hernia recurrence. More importantly, the study found that length of hospital stay, readmission rate, early adverse event rate, serious adverse event rate, and incidence of bloating or worsening of baseline bloating was lower in patients with cTIF compared to HH repair followed by LNF.[35]
Future. In addition to the current indication of TIF for primary GERD, it is also being explored in other clinical settings. First, it includes treatment of GERD after per-oral endoscopic myotomy (POEM). Initial feasibility was shown in five followed by three patients, where the authors found no serious adverse events and a resultant decrease in PPI use and esophagitis post-TIF.[36,37] Second area of future exploration is in patients with extraesophageal GERD or atypical GERD symptoms. These patients have variable effectiveness to ARS, and a lack of response to PPI therapy is considered predictive of a lack of response to ARS.[38–40] A systematic review and meta-analysis by our group have found effectiveness in reducing subjective symptoms of atypical GERD in this patient population.[26] Clinical trials are underway to study both of these applications.
In patients with GERD and obesity, Shah et al.[41] successfully demonstrated a case of same-session TIF followed by endoscopic sleeve gastroplasty (ESG). Another application is in patients with obesity who will be undergoing laparoscopic sleeve gastrectomy (LSG). TIF does not incorporate much of the gastric fundus into the fundoplication, so it can be performed before LSG. Clinical trials are underway to study this application. On the other hand, TIF can be technically challenging post-LSG as the remaining fundus may not be adequate for the procedure. Unfortunately, the data on such cases are limited.[42] Lastly, compared to standard Nissen Fundoplication, TIF can be performed in patients with impaired esophageal motility.[43,44] It is one of the reasons TIF is being assessed for patients with GERD postlung transplant.[45]
Radiofrequency Antireflux Procedure (Stretta)
The Stretta device (Restech, Houston, TX) gained FDA approval in 2000 as an endoscopic treatment for GERD. Since then, >25 000 procedures have been conducted. The Stretta system includes a radiofrequency (RF) reusable generator and a single use RF-catheter. The catheter is introduced and positioned against the squamocolumnar junction with the help of a guide wire after measuring the distance of the gastroesophageal junction (GEJ) using an endoscope. The catheter has a balloon basket that extends needle electrodes into the muscular layer of the esophagus after inflation. The electrodes administer radiofrequency energy to the lower esophageal sphincter (LES). The delivery of radiofrequency energy occurs at four levels within the esophagus and at the gastric cardia. Throughout the procedure, a constant flow of chilled water is used for irrigation via an integrated irrigation pump in the RF generator to prevent any harm to the mucosal lining. The mechanism of action is not entirely understood, but it is proposed that by applying RF energy at LES and gastric cardia, it decreases tissue compliance and lower esophageal sphincter relaxation.[46]
Systematic reviews and meta-analyses have yielded conflicting findings concerning the effectiveness of the Stretta procedure. In 2015, Lipka et al.[47] specifically examined randomized controlled trials and reported that Stretta did not lead to significant alterations in esophageal acid exposure, quality of life, or the ability to discontinue proton pump inhibitors (PPIs). Conversely, in 2017, Fass et al.[48] meta-analyzed both randomized controlled trials (RCTs) and cohort studies and concluded that Stretta improves both subjective and objective outcomes for patients with GERD except LES basal pressure. However, long-term observational data has shown the durability of the procedure for improving quality of life and decreasing PPI use over 8–10 years.[49,50] Stretta is considered one of the safest and easiest to perform among antireflux procedures. In a systematic review, the adverse event rate was found to be less than 1% among 2468 Stretta procedures performed.[48]
Fundoplication vs. Stretta. Over fundoplication, Stretta is relatively easier to perform and can have consistent reproducibility. It does not significantly affect the surgical anatomy, which makes it easier to perform another subsequent procedure in patients with inadequate response. Additionally, it can be performed in an outpatient endoscopy suite with no requirement for general anesthesia. However, given inconsistent efficacy outcomes, recently, a multisociety consensus conference and guideline document recommend the benefit of fundoplication over Stretta in adult patients with GERD.
It is relevant to highlight that their recommendation also included a preference for Stretta over continued PPI use[5] (Table 1).
Generally, the ideal candidates for Stretta include patients with confirmed GERD, having typical GERD symptoms (heartburn and/or regurgitation), low-grade erosive esophagitis (LA grade A and B), without Barrett's esophagus, small hiatal hernia, with a competent lower esophageal sphincter (LES pressure >5 mmHg) and having some response to PPI treatment.[51]
Future. GERD is a common postoperative adverse event in patients undergoing LSG. Given there is no anatomical limitation of the amount of fundus left post-LSG, as in the case of fundoplication, Stretta can potentially be performed in this patient cohort. Clinical trials are underway to study this application. However, a report from a small study that included 15 patients post-LSG showed no improvement in GERD symptoms or patient satisfaction despite a decrease in PPI use.[52] Another potential research area is using Stretta in patients with failed laparoscopic Nissen fundoplication (LNF). In a small subset of patients who underwent LNF, Stretta has improved subjective symptoms.[53,54] In short, postsurgical use of Stretta is technically possible but needs larger studies for the proof of concept. Overall, Stretta does not address all aspects of the anatomical pathology behind GERD, so its utilization will be limited to a niche population.
Endoscopic Suturing and Plication for Gastroesophageal Reflux Disease
Endoscopic suturing and plication techniques for GERD have evolved over the years since the 1980s.[55] The OverStitch device (Apollo Endosurgery, Austin, TX), first approved by the FDA in 2008, enables full-thickness sutures through a flexible endoscope. It is a multipurpose device for advanced endoscopic procedures, including endoscopic augmentation of the gastroesophageal junction for GERD. Recent modifications of the techniques include mucosal inflammatory response before suturing or plication for better tissue adherence. These include:
Mucosal Ablation and Suturing of EG Junction. The mucosal ablation and suturing of esophagogastric junction (EG) (MASE) procedure involves argon plasma (APC) coagulation before suturing below the EG junction. In pilot studies, MASE procedure has shown effectiveness in improving subjective symptoms of GERD and PPI usage for patients with altered anatomy.[56,57]
Resection and Plication. The resection and plication (RAP) procedure involves mucosal resection before full-thickness plication of the LES and cardia. In pilot studies, the RAP procedure has also shown effectiveness in improving subjective symptoms of GERD and PPI usage in patients with altered anatomy.[58,59] Compared to MASE, which consists of 2–3 interrupted sutures, the RAP procedure utilizes a single-running suture pattern.
More research is needed to determine the effectiveness and safety profile for MASE and RAP procedures. These procedures are primarily offered to patients with altered anatomy with limited antireflux surgery options. They offer the advantage of performing the procedure in antegrade without requiring retroflexion in this patient population with challenging anatomy.[45]
Curr Opin Gastroenterol. 2023;39(5):381-389. © 2023 Lippincott Williams & Wilkins