Abstract and Introduction
Abstract
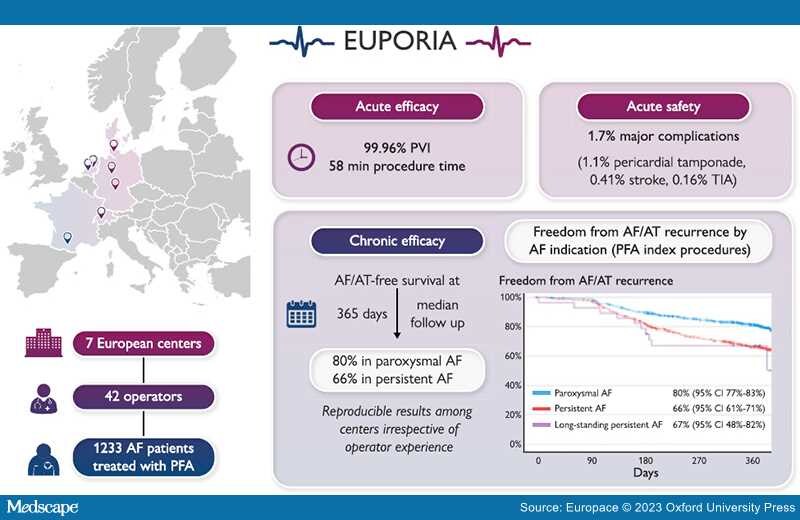
Aims: Pulsed field ablation (PFA) is a new, non-thermal ablation modality for pulmonary vein (PV) isolation in patients with atrial fibrillation (AF). The multi-centre EUropean Real World Outcomes with Pulsed Field AblatiOn in Patients with Symptomatic AtRIAl Fibrillation (EU-PORIA) registry sought to determine the safety, efficacy, and learning curve characteristics for the pentaspline, multi-electrode PFA catheter.
Methods and Results: All-comer AF patients from seven high-volume centres were consecutively enrolled. Procedural and follow-up data were collected. Learning curve effects were analysed by operator ablation experience and primary ablation modality. In total, 1233 patients (61% male, mean age 66 ± 11years, 60% paroxysmal AF) were treated by 42 operators. In 169 patients (14%), additional lesions outside the PVs were performed, most commonly at the posterior wall (n = 127). Median procedure and fluoroscopy times were 58 (interquartile range: 40–87) and 14 (9–21) min, respectively, with no differences due to operator experience. Major complications occurred in 21/1233 procedures (1.7%) including pericardial tamponade (14; 1.1%) and transient ischaemic attack or stroke (n = 7; 0.6%), of which one was fatal. Prior cryoballoon users had less complication. At a median follow-up of 365 (323–386) days, the Kaplan–Meier estimate of arrhythmia-free survival was 74% (80% for paroxysmal and 66% for persistent AF). Freedom from arrhythmia was not influenced by operator experience. In 149 (12%) patients, a repeat procedure was performed due to AF recurrence and 418/584 (72%) PVs were durably isolated.
Conclusion: The EU-PORIA registry demonstrates a high single-procedure success rate with an excellent safety profile and short procedure times in a real-world, all-comer AF patient population.
Graphical Abstract
Introduction
Atrial fibrillation (AF) is a growing global epidemic with substantial health economic burden. Increased awareness, advanced detection, and life expectancy contribute to the growing number of AF patients. An estimated 14–17 million Europeans will suffer from AF by 2030, and the expected number of new cases of AF per year will be 120 000–215 000.[1] Patients with AF have an increased risk of stroke, morbidity, and hospitalization, which places significant strain on an already overburdened healthcare system,[2] demonstrating the need for effective, safe, and readily available therapies.
Recent pivotal studies demonstrated catheter ablation as an effective first-line therapy in AF treatment[3–5] and as a means to slow AF progression.[6] With growing AF prevalence, the increased demand on electrophysiology labs necessitates continuous advancements in safe, effective, and efficient AF treatment strategies that allow for seamless adoption in clinical practice.[7]
Pulsed field ablation (PFA) is a new ablation modality for cardiac arrhythmias. Myocardium is characterized by a high susceptibility towards PFA in comparison to surrounding tissue.[8–10] This opens a broad therapeutic window composed of high efficacy (myocardial damage) with little to no collateral damage. Pulsed field ablation 'tissue selectivity' was confirmed in pre-clinical and clinical studies showing low vulnerability of nerves, vasculature, and oesophageal tissue to PFA.[11–16]
A dedicated 'single shot' pulmonary vein isolation (PVI) device that obtained CE mark in Europe in January 2021 was the Farapulse™ PFA ablation system (Boston Scientific, Menlo Park, CA, USA). Since its commercial release, the pentaspline, multi-electrode PFA catheter has shown encouraging acute efficacy and safety profiles.[13,17–22] Feasibility studies and early single-centre experiences have demonstrated lesion durability, safety, and initial long-term outcomes.[13,17,19–25] However, real-world outcomes in large patient populations are still scarce.[18] Chronic data are needed to further evaluate the use of this novel technology in a real-world setting and understand the learning curve. The aim of this registry is to describe real-world adoption, workflow, and acute and long-term outcomes after PFA in AF patients in high-volume European centres.
Europace. 2023;25(7):euad185 © 2023 Oxford University Press
Copyright 2007 European Heart Rhythm Association of the European Society of Cardiology (ESC). Published by Oxford University Press. All rights reserved.